[摘要]背景與目的:新的中心體調控蛋白Sec23同係物B(Sec23 homolog B,SEC23B)可以調節細胞中的自噬,從而提供有助於腫瘤生長和遠處轉移的能量和營養。然而,這種作用是否參與乳腺癌的浸潤轉移尚不清楚。本研究旨在探討SEC23B在乳腺癌浸潤轉移中的作用及其分子機製。方法:使用Kaplan-Meier Plotter數據庫和HCMDB數據庫分析SEC23B表達與乳腺癌預後、轉移之
[摘要]背景與目的:新的中心體調控蛋白Sec23同係物B(Sec23 homolog B,SEC23B)可以調節細胞中的自噬,從而提供有助於腫瘤生長和遠處轉移的能量和營養。然而,這種作用是否參與乳腺癌的浸潤轉移尚不清楚。本研究旨在探討SEC23B在乳腺癌浸潤轉移中的作用及其分子機製。方法:使用Kaplan-Meier Plotter數據庫和HCMDB數據庫分析SEC23B表達與乳腺癌預後、轉移之間的關聯。將MCF-7細胞分為載體組(control)和SEC23B過表達質粒組(SEC23B),以及將MDA231-LM2細胞分為SEC23B敲低組(sh-SEC23B)和對照組(sh-NC)。采用蛋白質印跡法(Western blot)檢測乳腺癌細胞中SEC23B、自噬相關蛋白[死骨片1(SQSTM1,p62)、微管相關蛋白1的輕鏈3(microtubule-associated-protein light-chain-3,LC3)]和細胞外調節蛋白激酶(extracellular-regulated protein kinases,ERK)/哺乳動物雷帕黴素靶蛋白(mammalian target of rapamycin,mTOR)信號轉導通路蛋白的表達。通過transwell實驗和傷口愈合實驗評估細胞遷移,采用免疫熒光染色檢測細胞中自噬顆粒。此外,構建體內腹膜內腫瘤模型研究SEC23B體內對乳腺癌細胞轉移的影響。結果:SEC23B高表達的乳腺癌患者的總生存期明顯短於SEC23B低表達的患者。SEC23B在轉移性乳腺癌中的表達明顯高於沒有轉移的乳腺癌原發性乳腺癌細胞(MCF-7、BT-549、MDA-MB-468和MDA-MB-453)中的SEC23B蛋白水平低於轉移性乳腺癌細胞(MDA231-LM2和ZR-75-30)。與control組相比,SEC23B組明顯加速了細胞遷移(P<0.001)。與sh-NC組相比, sh-SEC23B組明顯降低了細胞遷移(P<0.001)。與control組相比,SEC23B組LC3A/B的表達水平和自噬顆粒的形成明顯增加,而p62蛋白表達和mTOR、ERK的磷酸化水平降低。與sh-NC組相比,sh-SEC23B組LC3A/B表達水平和自噬顆粒的形成顯著降低,和mTOR、ERK的磷酸化水平升高,特別是在饑餓條件下結果更顯著。在體內實驗中,與sh-NC組相比,sh-SEC23B組腫瘤重量顯著降低(P<0.01),並且小鼠腫瘤組織中壞死組織增多和肺組織腫瘤轉移減少。sh-SEC23B組小鼠肺轉移腫瘤數和LC3免疫組織化學染色明顯低於sh-NC組(P<0.01)。結論:SEC23B通過抑製ERK/mTOR信號轉導通路來誘導乳腺癌自噬,並促進癌細胞轉移。
[關鍵詞]Sec23同係物B;乳腺癌;自噬;轉移;ERK/mTOR信號轉導通路
[Abstract]Background and purpose:The novel centrosomal regulatory protein Sec23 homolog B (SEC23B) regulates autophagy in cells, thereby providing energy and nutrients that contribute to tumor growth and distant metastasis. However, whether this role is involved in the mechanism of infiltrative metastasis in breast cancer is unclear. The aim of this study was to investigate the molecular mechanism by which SEC23B promotes breast cancer infiltration and metastasis.Methods:The association between SEC23B expression and breast cancer prognosis and metastasis was analyzed using Kaplan-Meier Plotter database and HCMDB database. MCF-7 cells were divided into vector group (control) and SEC23B overexpression plasmid group (SEC23B), and MDA231-LM2 cells were divided into SEC23B knockdown group (sh-SEC23B) and control group (sh-NC). Western blot was used to detect the expressions of SEC23B, autophagy-associated proteins [dead bone fragment 1 (SQSTM1, p62), microtubule-associated-protein light-chain-3 (LC3)] and extracellular-regulated protein kinases (ERK)/mammalian target of rapamycin (mTOR) pathway proteins in breast cancer cells. Cell migration was assessed by transwell assay and wound healing assay, and autophagic granules in cells were detected by immunofluorescence staining. In addition, an in vivo intraperitoneal tumor model was constructed to study the effect of SEC23B on breast cancer cell metastasis in vivo.Results:The overall survival of breast cancer patients with high SEC23B expression was significantly shorter than that of patients with low SEC23B expression. The expression of SEC23B in metastatic breast cancer was significantly higher than that in breast cancer without metastasis. SEC23B protein levels were lower in primary breast cancer cells (MCF-7, BT-549, MDA-MB-468 and MDA-MB-453) than in metastatic breast cancer cells (MDA231-LM2 and ZR-75-30). Compared with the control group, the SEC23B group significantly accelerated cell migration (P<0.001). Compared with the sh-NC group, the sh-SEC23B group significantly reduced cell migration (P<0.001). Compared with the control group, the expression level of LC3A/B and the formation of autophagy particles in the SEC23B group were significantly increased, while the expression of p62 protein and the phosphorylation levels of mTOR and ERK were decreased. Compared with the sh-NC group, the LC3A/B expression level and the formation of autophagy particles were significantly decreased in the sh-SEC23B group, and the phosphorylation levels of mTOR and ERK were increased, especially under starvation conditions. In in vivo experiments, tumor weight was significantly lower in the sh-SEC23B group compared with the sh-NC group (P<0.01), and there was an increase in necrotic tissue and a decrease in tumor metastasis in lung tissue in mice. sh-SEC23B group mice had a significantly lower number of lung metastatic tumors and LC3 immunohistochemical staining than the sh-NC group (P<0.01).Conclusions:SEC23B induces breast cancer autophagy and promotes cancer cell metastasis by inhibiting ERK/mTOR signaling pathway.
[Key word]Sec23 homolog B; Breast cancer; Autophagy; Metastasis; ERK/mTOR signaling pathway
乳腺癌是女性中最常見的腫瘤,並且轉移是乳腺癌患者死亡的主要原因[1]。腫瘤轉移是一個連續的、多步驟的病理學過程,需要細胞內外蛋白質共同參與[2]。在真核生物中,蛋白質分泌途徑由一組細胞器和載貨囊泡組成,該途徑的第一步是由外殼蛋白Ⅱ(coat protein Ⅱ,COPⅡ)複合物將蛋白質從內質網轉運到高爾基體[3]。Sec23同係物B(Sec23 homolog B,SEC23B)是COPⅡ複合物中的一種重要成分,它在COPⅡ組裝和囊泡出芽中起作用,並參與調節蛋白質和脂質從內質網到細胞中高爾基體的運輸[4]。先前的研究[5-7]表明,SEC23B異常激活可能會誘發癌症,並與甲狀腺癌、肝細胞癌和前列腺癌的發展有關。最近研究[8]發現,SEC23B可以幫助調節細胞中的自噬。自噬是維持細胞穩態、調節細胞信號轉導和促進細胞存活的關鍵細胞降解過程[9]。氧化應激在實體瘤中會誘導自噬,從而提供有助於腫瘤生長和遠處轉移的能量和營養[10]。因此,SEC23B是否通過介導自噬誘導腫瘤轉移值得進一步研究。為了係統地探索SEC23B在自噬和腫瘤轉移中的作用以及導致這些過程的潛在機製,我們進行了SEC23B的過表達或敲低實驗來調節乳腺癌細胞中SEC23B表達,以探討其對自噬、轉移的影響。此外,我們使用了體內異種移植小鼠模型來證實SEC23B介導的自噬對乳腺癌細胞轉移的影響。
1材料和方法
1.1 細胞係、試劑和儀器
人乳腺癌細胞係MCF-7接種在DMEM培養基中培養,人乳腺癌細胞係MDA-MB-468和MDA-MB-453在Leibovitz's L-15中培養,人乳腺癌細胞係BT-549、ZR-75-30和MDA231-LM2在RPMI-1640(上述材料均購自上海源培生物科技股份有限公司)中培養。在37℃、CO2體積分數為5%的環境下,所有培養基均添加10%胎牛血清(fetal bovine serum,FBS)(購自美國Gibco公司)和50 IU青黴素/鏈黴素(購自上海源培生物科技股份有限公司)。
LipofectamineTM3000購自美國Invitrogen公司,針對SEC23B、肌動蛋白的一抗購自美國Proteintech公司,針對細胞外調節蛋白激酶(extracellular-regulated protein kinases,ERK)、p-ERK、哺乳動物雷帕黴素靶蛋白(mammalian target of rapamycin,mTOR)、p-mTOR、微管相關蛋白1的輕鏈3(microtubule-associated protein light-chain-3,LC3)A/B、死骨片1(SQSTM1,p62)的一抗購自美國CST公司,針對GAPDH的一抗購自美國Transgene公司,transwell板購自美國Corning公司,BCA檢測試劑盒購自北京鼎國昌盛生物技術有限責任公司。
LAS 4000化學發光成像分析儀購自美國GE Healthcare公司,SP5共聚焦激光掃描顯微鏡購自德國Leica公司。
1.2 臨床數據庫分析
從Kaplan-Meier Plotter數據庫中(https://kmplot.com/analysis/)獲得2 477例SEC23B低表達和2 452例SEC23B高表達乳腺癌患者的生存數據分析比較。利用HCMDB數據庫(https://hcmdb.i-sanger.com)箱線分析368例乳腺癌患者SEC23B表達與乳腺癌轉移之間的關聯。
1.3 細胞分組和轉染
為了考察SEC23B對乳腺癌細胞遷移和自噬的影響,將MCF-7細胞轉染SEC23B過表達質粒(SEC23B)來上調SEC23B表達,將MDA231-LM2細胞轉染靶向SEC23B的化學修飾Stealth短發夾RNA(sh-SEC23B)以敲低SEC23B表達。使用LipofectamineTM3000將SEC23B、sh-SEC23B1或對照轉染細胞48 h。
1.4 shRNA、質粒和轉染
將細胞以3×104個細胞接種在6 cm培養皿中,並在不含抗生素的培養基中培養24 h。靶向SEC23B的化學修飾Stealth短發夾RNA(sh-SEC23B)和對照(sh-NC),以及Flag-SEC23B質粒編碼N末端帶有Flag標記的SEC23B蛋白(SEC23B)和對照(control)購自廣州Ribobio公司。根據產品說明書,使用LipofectamineTM3000將siRNA和質粒轉染到細胞中。用終濃度為20 nmol/L的siRNA轉染細胞。在轉染48 h後,收集細胞通過蛋白質印跡法(Western blot)分析轉染效率。
1.5 Western blot檢測
細胞用PBS洗滌兩次,並置於RIPA裂解緩衝液[150 mmol/L NaCl、0.1% SDS、50 mmol/L Tris-HCl (pH為7.5)、1% NP-40、1%脫氧膽酸鈉]中,在冰上裂解20 min。使用BCA檢測試劑盒測量每個樣品的蛋白質濃度。隨後,將聚丙烯酰胺凝膠電泳(polyacrylamide gel electrophoresis,PAGE)分離的蛋白質轉移到硝酸纖維素膜上,然後分別與各種抗體一起溫育。用於Western blot檢測的抗體用含有0.01%疊氮化鈉和5%牛血清白蛋白的緩衝液以不同比例稀釋。使用LAS 4000化學發光成像分析儀令Western blot檢測可視化。使用的抗體包括針對SEC23B(1∶500)、p-ERK(1∶500)、ERK(1∶500)、p-mTOR(1∶800)、mTOR(1∶800)、LC3A/B(1∶500)、p62(1∶500)、肌動蛋白(1∶5 000作為陰性對照)和GAPDH(1∶6 000作為陰性對照)的一抗。所有Western blot檢測進行3次進行。用Image J軟件對條帶強度進行量化。
1.6 Transwell檢測
將2×104細胞用無血清DMEM洗滌,懸浮在0.2 mL無血清DMEM中,並添加到transwell小室的上層(8 μm孔徑,24 孔板)。隨後將0.5 mL含有20%FBS的DMEM添加到下室中。將腔室在37℃下溫育24 h後,將濾膜用甲醇固定10 min,並用0.1%結晶紫染色15 min。用棉簽輕輕去除上室的細胞。在顯微鏡下以100×放大倍數捕獲下室遷移的細胞,並在5個隨機視野中計數。每個實驗至少重複3次。遷移率標準化為對照組。
1.7 劃痕試驗
在刮擦前一天將細胞接種在6孔板中,以獲得大約100%的細胞密度。用200 μL移液器吸嘴進行直劃痕,然後用PBS洗滌細胞2次並用RPMI-1640培養基培養。每12 h記錄1次劃痕的長度。
1.8 免疫熒光染色
載玻片上的細胞在室溫下用4%多聚甲醛固定15 min。隨後,用PBS衝洗兩次後,用0.4% Triton-X100透化細胞30 min,並在室溫下用5%BSA封閉1 h,然後將細胞與抗GFP-LC3的一抗按1∶100比例在4℃下溫育過夜,與二抗在室溫下溫育1 h,然後用DAPI複染。使用SP5共聚焦激光掃描顯微鏡獲取圖像,並使用Image J進行分析。
1.9 異種移植瘤模型
無特定病原體(SPF)級雌性BALB/c裸小鼠(15~20 g,6~8周齡)購自湖南斯萊克景達實驗動物有限公司(許可號:SCXK 2019-0004)。所有小鼠都安置在平均恒溫(23±2℃)的房間中,光照周期為12 h,相對濕度為50%~60%,並且可以自由獲取標準顆粒飼料和水。實驗前將小鼠在這些環境中適應性喂養至少1周。將所有小鼠隨機分為2組(n=6):sh-NC組和sh-SEC23B組。將具有穩定SEC23B敲低(sh-SEC23B)或陰性對照(sh-NC)的MDA231-LM2細胞(1×106個細胞)腹膜內注射到小鼠中。3周後,將小鼠在麻醉下處死。切除腫瘤和肺組織,分別在4%多聚甲醛和布因氏液中固定,用於進一步分析。
1.10 H-E染色和免疫組織化學染色
將腫瘤和肺樣本包埋在石蠟中以製備4 μm厚的切片,用蘇木精和伊紅(hematoxylin and eosin,H-E)染色,並在光學顯微鏡下觀察。對於免疫組織化學染色,將腫瘤切片用二甲苯脫蠟並用梯度乙醇水化,然後使用0.01 mol/L檸檬酸鹽緩衝液進行抗原和抗體修複。切片用3% H2O2處理,然後在室溫下用10%山羊血清封閉30 min。LC3(1∶200)一抗在4℃溫育過夜,二抗在室溫溫育30 min。使用DAB進行顯色後,將載玻片用蘇木精複染2 min,然後在光學顯微鏡下觀察。
1.11 統計學處理
所有數據均使用SPSS 21.0進行處理。連續性變量數據描述為x±s,兩組間比較使用Student’st檢驗(雙尾)檢驗分析實驗結果,多組間比較采用單因素方差分析方法分析實驗結果。P <0.05為差異有統計學意義。
2結 果
2.1 SEC23B在轉移性乳腺癌中上調
為了評估SEC23B在乳腺癌中的作用,通過Kaplan-Meier分析了SEC23B表達水平與乳腺癌患者存活率之間的相關性。SEC23B高表達的乳腺癌患者的總生存期明顯短於SEC23B低表達的患者(圖1A)。此外,在臨床數據庫HCMDB中分析了有或無轉移的乳腺癌中的SEC23B表達。SEC23B在轉移性乳腺癌中的表達明顯高於沒有轉移的乳腺癌,表明SEC23B高表達是乳腺癌預後不良的標誌物。然後,在從原發性或轉移性乳腺癌中分離的可用乳腺癌細胞係中分析SEC23B蛋白水平(圖1B)。乳腺癌細胞係(MCF-7、BT-549、MDA-MB-468和MDA-MB-453)中的SEC23B蛋白水平低於高轉移潛能乳腺癌細胞係(MDA231-LM2和ZR-75-30,圖1C)。

圖1 SEC23B在轉移性肝癌中表達上調
Fig. 1 SEC23B is upregulated in metastatic breast cancer
A:Kaplan-Meier survival analysis of the correlation between SEC23B expression levels and overall survival of breast cancer patients was performed in an online database (n=4 929);B:Association between SEC23B expression and breast cancer metastasis was analyzed using the HCMDB database box line (n=368);C:SEC23B expression in breast cancer cells was detected by Western blot.
2.2 SEC23B在體外促進乳腺癌的遷移
為了進一步研究SEC23B在乳腺癌中的功能,在MCF-7細胞中分析了過表達SEC23B對細胞遷移的影響(圖2A)。Transwell和劃痕實驗結果顯示,與control組相比,SEC23B組明顯加速了細胞遷移和傷口愈合(P<0.001,圖2B、C)。此外,在MDA231-LM2細胞中敲低了SEC23B(圖2D),與sh-NC組相比,sh-SEC23B組明顯降低了細胞遷移和傷口愈合(P<0.001,圖2E、F)。
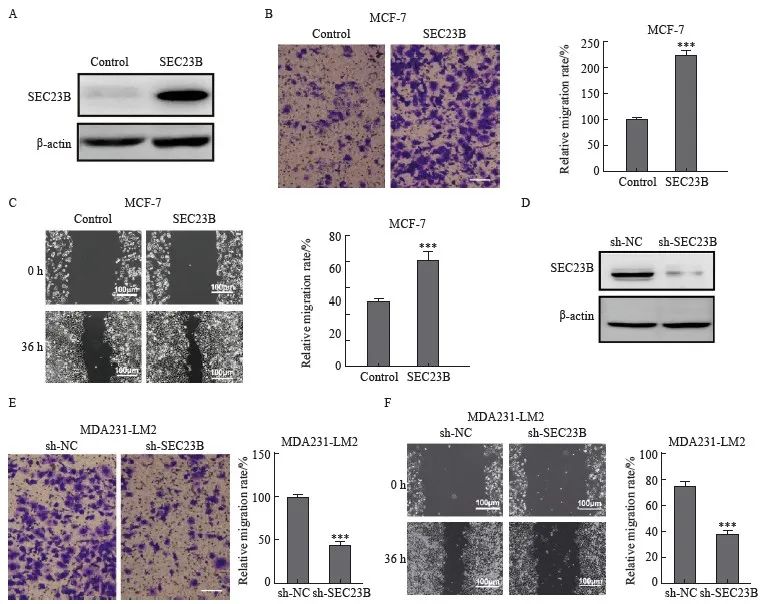
圖2 SEC23B在體外促進乳腺癌細胞的遷移
Fig. 2 SEC23B promotes breast cancer migration in vitro
A:Transfection efficiency of SEC23B overexpression plasmid in MCF-7 cells was examined by Western blot;B-C:Effect of SEC23B overexpression on MCF-7 cell migration was assessed by transwell assay and wound healing assay;D:The expression of sh-SEC23B lentivirus in MDA231-LM2 cells by Western blot;E-F:Effect of SEC23B knockdown on migration of MDA231-LM2 cells was assessed by transwell assay and wound healing assay.***:P<0.001, compared to control or sh-NC groups.
2.3 SEC23B誘導乳腺癌細胞自噬
為了探索SEC23B是否參與自噬,本研究在抑製或過表達SEC23B後檢測了乳腺癌細胞係MCF-7和MDA231-LM2中自噬標誌物LC3的變化。與control組相比,SEC23B組LC3A/B的表達水平增加,而p62蛋白表達降低(圖3A)。免疫熒光實驗結果顯示,SEC23B組自噬顆粒的形成較control組顯著增加(P<0.01)(圖3B)。相反,與sh-NC組相比,sh-SEC23B組LC3A/B表達水平和自噬顆粒的形成降低,在饑餓條件下更為顯著(P <0.01,圖3C、D)。結果表明,SEC23B可促進乳腺癌細胞中的自噬。本研究進一步分析了抑製自噬對乳腺癌細胞遷移的影響,采用自噬抑製劑CQ處理過表達SEC23B的MCF-7細胞,結果顯示,與PBS組相比,CQ組細胞遷移顯著降低(P <0.001,圖3E)。
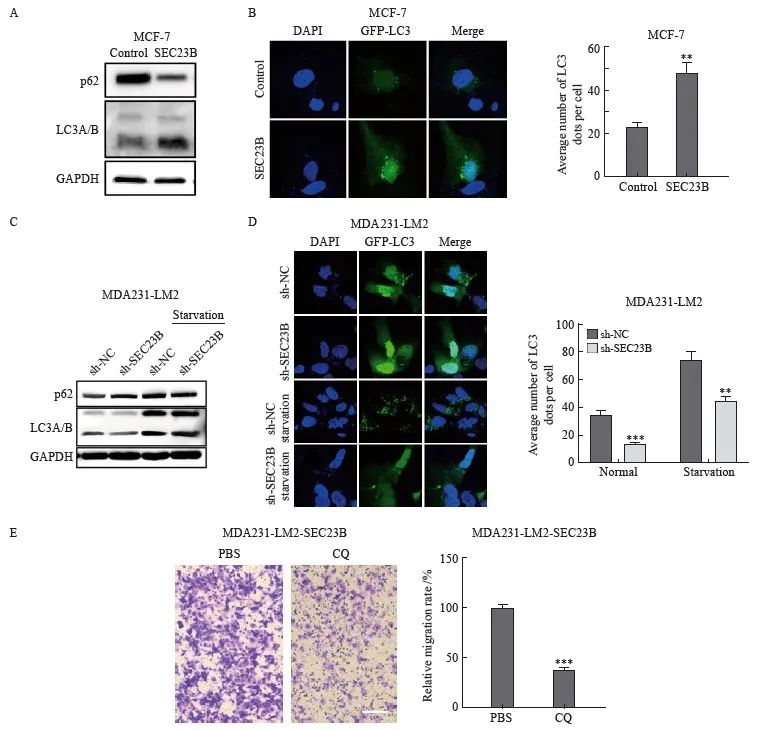
圖3 SEC23B誘導乳腺癌細胞自噬
Fig. 3 SEC23B induces autophagy in breast cancer cells
A:Effect of SEC23B overexpression plasmid on p62, LCA/B expression in MCF-7 cells detected by Western blot; B: Representative images and quantitative analysis of the effect of SEC23B overexpression on autophagic granules in MCF-7 cells detected by immunofluorescence staining;C:Effect of SEC23B knockdown on p62, LCA/B expression in MDA231-LM2 cells by Western blot;D:Representative images and quantitative analysis of the effect of SEC23B knockdown on autophagic granules in MDA231-LM2 detected by immunofluorescence staining;E:Effect of CQ on migration of SEC23B overexpressing MCF-7 cells was assessed by transwell assay.**:P<0.01, compared with control or sh-NC groups;***:P<0.001, compared with control or sh-NC groups.
2.4 ERK/mTOR信號轉導通路參與SEC23B在乳腺癌細胞中誘導的自噬
ERK/mTOR信號轉導通路是影響自噬的關鍵因素,因此我們推測該信號轉導通路可能參與了SEC23B在乳腺癌細胞中誘導的自噬。SEC23B上調後,MCF-7細胞中mTOR和ERK的磷酸化水平降低(圖4A)。相反,SEC23B敲低後,MDA231-LM2細胞中mTOR和ERK的磷酸化水平升高,特別是在饑餓條件下(圖4B)。以上結果表明ERK/mTOR信號轉導通路可能參與SEC23B誘導的乳腺癌細胞自噬。為了進一步證明SEC23B通過ERK/mTOR信號轉導通路影響自噬,我們用ERK激動劑表皮生長因子(epidermal growth factor,EGF)處理SEC23B過表達的MCF-7細胞,結果顯示, EGF抑製了SEC23B誘導的LC3A/B和p62的蛋白表達水平變化(圖4C)。免疫熒光實驗結果顯示,與SEC23B組相比,SEC23B+EGF組的自噬顆粒形成顯著降低(P <0.05,圖4D)。這些結果表明,SEC23B通過影響ERK/mTOR信號轉導通路來誘導乳腺癌細胞自噬。
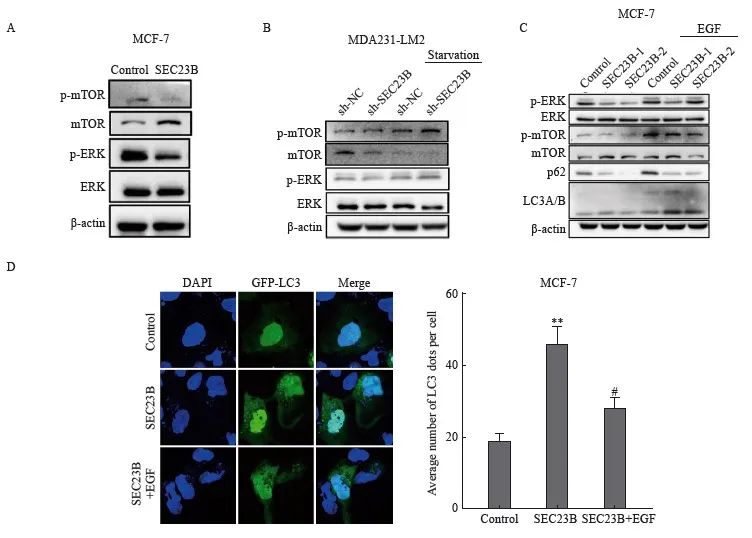
圖4 ERK/mTOR信號轉導通路參與SEC23B在乳腺癌細胞中誘導的自噬
Fig. 4 ERK/mTOR pathway is involved in SEC23B-induced autophagy in breast cancer cells
A:Effect of SEC23B overexpression plasmid on ERK/mTOR pathway expression in MCF-7 cells detected by Western blot;B:Effect of SEC23B knockdown on ERK/mTOR pathway expression in MDA231-LM2 cells detected by Western blot;C:Effect of EGF on ERK/mTOR pathway expression in MCF-7 cells detected by Western blot, effects of EGF on ERK/mTOR pathway and p62, LCA/B expression in SEC23B overexpressing MCF-7 cells detected by Western blot;D:Representative images and quantitative analysis of the effects of EGF on autophagic granules in SEC23B overexpressing MCF-7 cells detected by immunofluorescence staining.**:P<0.01, compared with the control group;#:P<0.05, compared with the SEC23B group.
2.5 SEC23B敲低在體內抑製乳腺癌轉移和自噬
為了進一步驗證SEC23B對移植瘤的影響,小鼠皮下注射了具有穩定SEC23B敲低(sh-SEC23B)或陰性對照(sh-NC)的MDA231-LM2細胞。與sh-NC組相比,sh-SEC23B組腫瘤體積更小,並且腫瘤重量顯著降低(P<0.01,圖5A)。H-E染色結果顯示,與sh-NC組相比,sh-SEC23B組小鼠腫瘤組織中壞死組織增多(圖5B),並且肺組織腫瘤轉移減少(圖5C)。取肺組織進行Bouin固定,sh-SEC23B組小鼠肺轉移腫瘤數明顯低於sh-NC組(P<0.01,圖5D)。此外,sh-SEC23B組小鼠腫瘤組織中LC3免疫組織化學染色明顯低於sh-NC組(P <0.001,圖5E)。結果表明,SEC23B可以作為致癌基因在體內促進乳腺癌細胞生長、轉移和自噬。
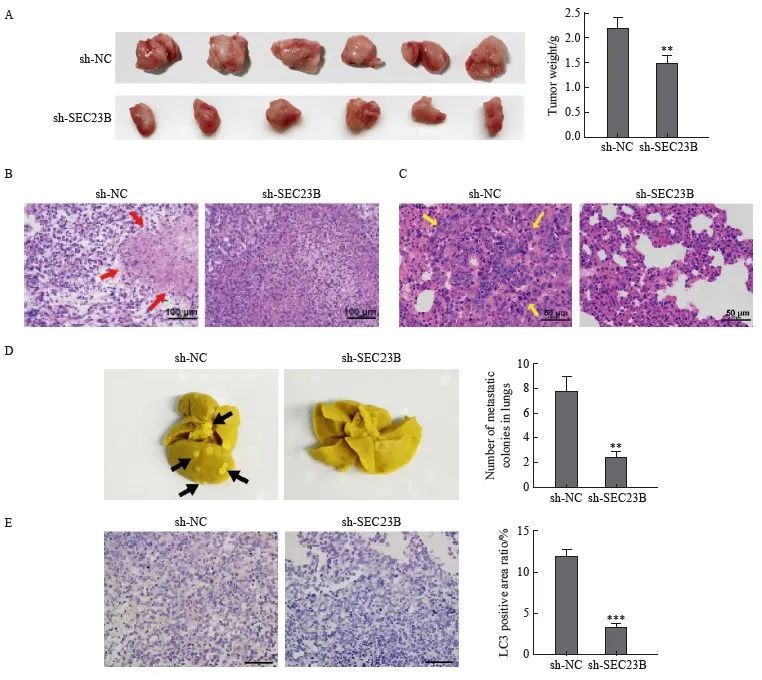
圖5 SEC23B敲低在體內抑製乳腺癌轉移和自噬
Fig. 5 SEC23B knockdown inhibits breast cancer metastasis and autophagy in vivo
A:Tumors were excised 21 days after implantation and final tumor weight was recorded. B: Histological examination by H-E staining was performed to determine tumor morphology and structural changes. Tumor necrosis was indicated by a red arrow.C:Lung metastases were examined by H-E staining. Tumor metastases in lung tissue were indicated by yellow arrows.D:Lung tissue was excised and fixed in Bouin solution 21 days after implantation, and the number of metastatic nodules was recorded for each mouse. Metastatic nodules were indicated by black arrows.E:LC3 expression in tumor tissues was examined by H-E staining.**:P<0.01, compared with sh-NC group;***:P<0.001, compared with sh-NC group.
3討 論
本研究探討了SEC23B在促進乳腺癌轉移中的潛在機製以及SEC23B在自噬中的潛在作用。數據庫分析結果顯示,SEC23B高表達的患者生存時間較短,並且與腫瘤轉移有關。在體外實驗中,SEC23B增強了乳腺癌細胞的遷移能力並誘導了自噬。我們的研究揭示了SEC23B促進乳腺癌進展和抑製自噬的新機製,這可能為乳腺癌治療提供新的見解。
SEC23B是一種GTP酶激活蛋白,可刺激SAR1-GTP水解以促進體內囊泡運輸[4]。研究[11]表明,SEC23B基因突變導致先天性紅細胞生成異常性貧血Ⅱ型。迄今為止,關於SEC23B異常表達與人類癌症發展的報道較少。研究[12]發現,SEC23B上調能夠促進正常甲狀腺細胞的增殖、集落形成、存活和侵襲,這與甲狀腺癌誘發的癌症易感性有關。另一項研究[13]表明,SEC23B通過核糖體生物合成途徑導致癌症易感性。此外,一項串聯質譜分析發現,SEC23B表達在肝腫瘤大鼠內質網中上調,提示SEC23B可用作肝細胞癌的潛在新型腫瘤標誌物[7]。有研究[6]證實SEC23B是miR-130a的靶標,後者在前列腺癌中下調,而敲低SEC23B表達模擬了miR-130a在前列腺癌細胞中過表達的影響。與這些研究報道的結果一致,本研究發現SEC23B過表達促進了乳腺癌細胞的轉移,而SEC23B沉默抑製了體內腫瘤的生長和轉移。這些結果證實SEC23B在乳腺癌的進展中起關鍵作用。
自噬在腫瘤發生或抑製中發揮雙重作用[14]。在腫瘤發生的早期,自噬作為一種腫瘤抑製因子,可以降解腫瘤細胞中的增殖相關蛋白和結構底物,並激活受損細胞中的程序性細胞死亡[15]。然而,隨著腫瘤的生長,氧化應激在實體瘤中誘導自噬,從而提供有助於腫瘤生長和遠處轉移的能量和營養[10]。相比之下, LC3B的高表達與腫瘤侵襲和轉移有關,並提示預後不良[16]。在本研究中,我們發現SEC23B促進了乳腺癌細胞中的自噬,並且用自噬抑製劑CQ處理過表達SEC23B的MCF-7細胞後,細胞轉移能力降低。研究[17]發現,營養缺乏會導致SEC23B重新定位於內質網高爾基體中間區,促進自噬通量。此外,還有研究[18]發現SEC23B參與細胞內COPⅡ囊泡的形成,這是自噬過程中自噬體生物發生的關鍵步驟。這些結果提示,SEC23B可能通過誘導乳腺癌細胞自噬促進癌細胞轉移。
自噬可以通過不同的信號轉導通路調節,尤其是mTOR信號轉導通路,它可以由MAPK/ERK信號轉導通路調節[19]。本研究發現SEC23B抑製了乳腺癌細胞的ERK/mTOR信號轉導通路。SEC23B通過抑製PPP1CA介導的ERK/MAPK信號轉導通路促進結直腸癌的生長和轉移[20]。本
研究還發現ERK激動劑EGF處理過表達SEC23B的MCF-7細胞後,細胞自噬水平降低。這些結果表明SEC23B至少部分地通過抑製ERK/mTOR信號轉導通路激活乳腺腫瘤細胞的自噬,進而促進腫瘤細胞遷移。然而,SEC23B如何抑製ERK/mTOR信號轉導通路的機製以及SEC23B的上遊信號轉導通路仍需進一步探索。
綜上所述,本研究發現SEC23B通過抑製ERK/mTOR信號轉導通路來誘導乳腺癌細胞自噬,並促進腫瘤細胞轉移。結果表明SEC23B可能是一種有應用前景的預後生物標誌物和乳腺癌治療的潛在治療靶點。然而,由於本研究沒有納入臨床病例分析SEC23B在乳腺癌組織中的表達情況,並且分析SEC23B生存數據時隻是采用單因素分析,未來需要在臨床隊列中通過多因素模型來驗證本研究的結果。
利益衝突聲明:所有作者均聲明不存在利益衝突。
[參考文獻]
[1] 王 帥, 崔中豪, 楊 毅. HER2陽性乳腺癌腦轉移的靶向治療研究進展[J]. 醫學研究生學報, 2020, 33(2): 215-219.
WANG S, CUI Z H, YANG Y. Research progress of targetedtherapy for HER2 positive breast cancer with brain metastases[J]. J Med Postgrad, 2020, 33(2): 215-219.
[2] FARES J, FARES M Y, KHACHFE H H, et al. Molecularprinciples of metastasis: a hallmark of cancer revisited[J].Signal Transduct Target Ther, 2020, 5(1): 28.
[3] ARAKEL E C, SCHWAPPACH B. Formation of COPI-coatedvesicles at a glance[J]. J Cell Sci, 2018, 131(5): jcs209890.
[4] WEI W, LIU Z G, ZHANG C, et al. A common human missensemutation of vesicle coat protein SEC23B leads to growthrestriction and chronic pancreatitis in mice[J]. J Biol Chem,2022, 298(1): 101536.
[5] ZHOU J G, SINGH P, YIN K H, et al. Non-medullary thyroidcancer susceptibility genes: evidence and disease spectrum[J]. Ann Surg Oncol, 2021, 28(11): 6590-6600.
[6] RAMALHO-CARVALHO J, MARTINS J B, CEKAITE L, et al.Epigenetic disruption of miR-130a promotes prostate cancer bytargeting SEC23B and DEPDC1[J]. Cancer Lett, 2017, 385:150-159.
[7] LIU Z, WANG Q, MAO J W, et al. Comparative proteomicanalysis of protein methylation provides insight into theresistance of hepatocellular carcinoma to 5-fluorouracil[J]. JProteomics, 2020, 219: 103738.
[8] ZEYEN L, DÖRING T, STIELER J T, et al. Hepatitis B subviralenvelope particles use the COPII machinery for intracellulartransport via selective exploitation of Sec24A and Sec23B[J].Cell Microbiol, 2020, 22(6): e13181.
[9] 周 慧, 韓 翰, 周偉強. SAHA-CTSV軸通過誘導過度自噬抑製乳腺癌MCF-7細胞的生長[J]. 中國藥理學通報,2021, 37(4): 504-510.
ZHOU H, HAN H, ZHOU W Q. SAHA-CTSV inducedexcessive autophagy and inhibited growth of breast cancerMCF-7 cells[J]. Chin Pharmacol Bull, 2021, 37(4): 504-510.
[10] MAITI A, HAIT N C. Autophagy-mediated tumor cell survivaland progression of breast cancer metastasis to the brain[J]. JCancer, 2021, 12(4): 954-964.
[11] KING R, LIN Z S, BALBIN-CUESTA G, et al. SEC23A rescuesSEC23B-deficient congenital dyserythropoietic anemia type Ⅱ[J]. Sci Adv, 2021, 7(48): eabj5293.
[12] YEHIA L, NIAZI F, NI Y, et al. Germline heterozygous variantsin SEC23B are associated with cowden syndrome and enrichedin apparently sporadic thyroid cancer[J]. Am J Hum Genet,2015, 97(5): 661-676.
[13] YEHIA L, JINDAL S, KOMAR A A, et al. Non-canonicalrole of cancer-associated mutant SEC23B in the ribosomebiogenesis pathway[J]. Hum Mol Genet, 2018, 27(18): 3154-3164.
[14] PIFFOUX M, ERIAU E, CASSIER P A. Autophagy as atherapeutic target in pancreatic cancer[J]. Br J Cancer,2021, 124(2): 333-344.
[15] DOWER C M, WILLS C A, FRISCH S M, et al. Mechanisms andcontext underlying the role of autophagy in cancer metastasis[J]. Autophagy, 2018, 14(7): 1110-1128.
[16] FAN Q, YANG L, ZHANG X D, et al. Autophagy promotesmetastasis and glycolysis by upregulating MCT1 expression andWnt/β-catenin signaling pathway activation in hepatocellularcarcinoma cells[J]. J Exp Clin Cancer Res, 2018, 37(1): 9.
[17] JEONG Y T, SIMONESCHI D, KEEGAN S, et al. The ULK1-FBXW5-SEC23B nexus controls autophagy[J]. Elife, 2018,7: e42253.
[18] HUANG S F, TANG M Z, JIANG H L, et al. A COPII subunitinteracting with ER-phagy receptor: a new potential avenue tomaintaining neuronal homeostasis[J]. Acta Biochim BiophysSin (Shanghai), 2020, 52(6): 698-700.
[19] XU H Y, LIU L Y, DING M, et al. Effect of Ganodermaapplanatum polysaccharides on MAPK/ERK pathway affectingautophagy in breast cancer MCF-7 cells[J]. Int J BiolMacromol, 2020, 146: 353-362.
[20] YANG C Y, CHEN N, LI X, et al. Mutations in the coat complexⅡ component SEC23B promote colorectal cancer metastasis[J]. Cell Death Dis, 2020, 11(3): 157.
copyright©醫學論壇網 版權所有,未經許可不得複製、轉載或鏡像
京ICP證120392號 京公網安備110105007198 京ICP備10215607號-1 (京)網藥械信息備字(2022)第00160號